Abstract
Introduction
Systemic AL amyloidosis is characterised by deposition of misfolded immunoglobulin light chains within organs. Treatment for amyloidosis is generally derived from that for multiple myeloma (MM). Combinations of immunomodulatory drugs and proteasome inhibitors are standard frontline MM therapy, but there is little experience with such regimens in AL. For patients not receiving autologous stem cell transplantation (ASCT), bortezomib-based regimens have been first-line treatment in AL amyloidosis over the last few years. The purpose of this study is to investigate the efficacy of bortezomib-based regimens for patients with newly diagnosed AL amyloidosis
Methods
We performed a retrospective study of all newly diagnosed patients with AL amyloidosis treated at our center between 4/1/11 and 12/31/17. Data pertaining to demographics, diagnosis, treatment and follow-up were extracted from electronic medical records. Survival curves were constructed according to the Kaplan-Meier method and compared using the log rank test. All statistical analyses were performed by using the SPSS 24.0 software. Time to progression (TTP) is defined as time from the date of diagnosis or the start of treatment for a disease until the disease starts to get worse or spread to other parts of the body. The primary endpoint was overall response rate and secondary endpoints were overall survival (OS) and TTP.
Results
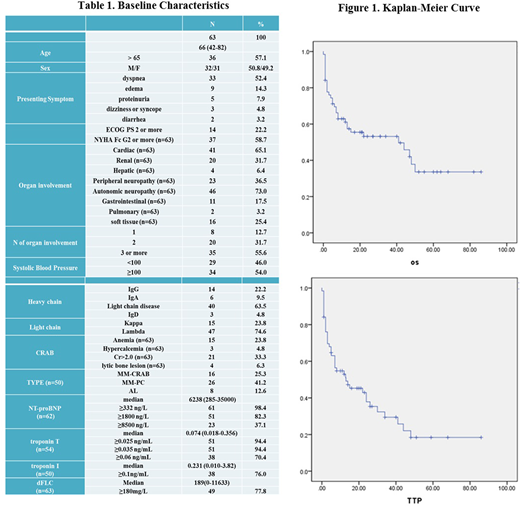
A total 63 patients with newly diagnosed AL amyloidosis who did not receive ASCT were analyzed. Clinical characteristics are shown in Table 1. They included 32 men and 31 women with a median age of 66 years (range, 42-82). Autonomic nerve, Cardiac, peripheral nerve, renal, soft tissue, and liver involvement were found in 46 (73%), 41 (65.1%), 23 (36.5%), 20 (31.7%), 16 (25.4%), 4(6.4%), respectively. The Mayo 2012 stage was: Stage 2 3.8%, Stage 3 30.8% and stage 4 65.4%. Hematological responses were: complete response (CR) 33.3 %, VGPR 19.0%, partial response (PR) 12.6% and no-response (NR) 17.4%. Organ response was 26.9% (n=17). With a median follow-up of 34 months, median OS was 40 months (95% CI 30-50) (Figure 1A) and median TTP was 27 months (95% CI 18-36) (Figure 1B). The rate of early death within 6 months was 28.5% (n=18). Patients were classified according to first-line treatment; bortezomib-based regimens (VMP, n=37; VD (bortezomib and dexamethasone), n=9; VCD, n=8; VMD, n=8; VTD (bortezomib with thalidomide and dexamethasone, n=1). Hematological responses of VMP, VD, VCD, VMD, and VTD were: 75.6%, 55.5%, 50.0%, 50.0%, 100%, respectively. Organ responses of VMP, VD, and VMD were: 35.1%, 22.2%, 25.0%, respectively.
Conclusions
In this retrospective analysis, bortezomib-based regimens in newly diagnosed AL amyloidosis showed results that appear comparable to those seen at other centers. These findings therefore continue to support the emerging roles for bortezomib-based regimens for the purposes of improving response. Larger scale analyses in multi-center trials would be beneficial to further study these roles.
Kim:Kyowa-Kirin: Research Funding; Roche: Research Funding; Mundipharma: Research Funding; Novartis: Research Funding; Celltrion: Research Funding; J&J: Research Funding; Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal